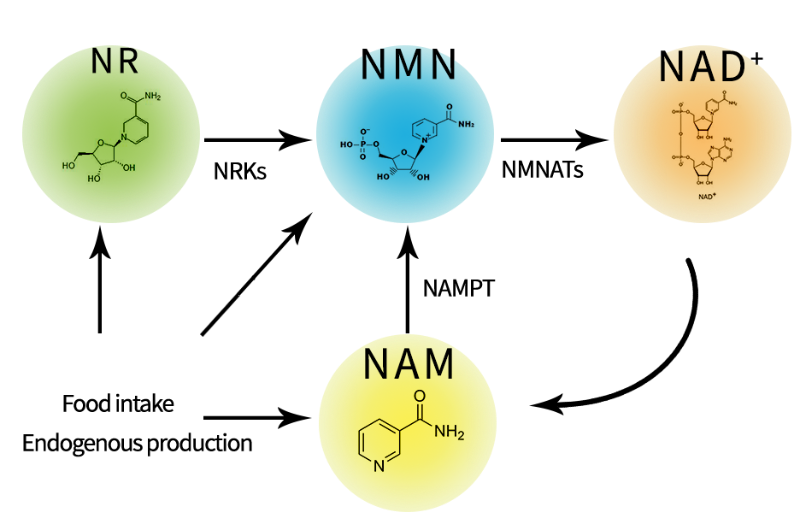
Nicotinamide Mononucleotide (NMN) has emerged as a prominent molecule in the global wellness sector, celebrated for its potential to counteract age-related physiological decline. As a precursor to Nicotinamide Adenine Dinucleotide (NAD+), a crucial coenzyme for cellular energy production and DNA repair, NMN’s rising popularity is rooted in its role in boosting declining NAD+ levels. Its application within the European market, however, presents a unique landscape of usage, regulatory challenges, and future trends.

Nicotinamide Mononucleotide Market Request
The primary domain of NMN use in Europe is the dietary supplement market, targeting health-conscious consumers seeking to promote healthy aging. Users typically ingest NMN orally in the form of capsules or sublingual powders, with daily dosages commonly ranging from 250mg to 500mg. The core rationale for its use is based on its proposed anti-aging and vitality-enhancing functions. Key purported benefits include enhancing cellular energy metabolism, which may translate to reduced fatigue and improved physical endurance. Furthermore, by supporting NAD+-dependent enzymes like sirtuins, NMN is believed to promote DNA repair, support cognitive health, and maintain cardiovascular function. Many users seek it for its potential to improve biomarkers of metabolic health, such as insulin sensitivity.
However, the legal status of Nicotinamide Mononucleotide NMN in Europe has recently undergone a significant shift, directly impacting the availability of legitimate NMN-containing health products. In 2022, the European Food Safety Authority (EFSA) assessed an application to approve NMN as a novel food. Based on the submitted evidence, EFSA concluded that NMN’s safety for use in food supplements was not established. Consequently, NMN is not authorized for sale as a food supplement within the European Union. This regulatory decision means that, unlike in the United States, it is increasingly difficult to find Nicotinamide Mononucleotide NMN in legally marketed over-the-counter health foods in mainstream EU channels. Any products containing NMN currently available are often sold in a regulatory grey area, marketed for “research purposes,” or sourced from outside the EU, posing questions about their quality and safety assurance.
Despite this regulatory hurdle, the future trajectory of NMN in Europe remains a subject of keen interest. The primary trend will be the generation of more robust scientific data. The current body of evidence, while promising, relies heavily on pre-clinical studies and small-scale human trials. Large-scale, long-term human clinical trials are essential to conclusively validate its efficacy and safety profile, which could potentially lead to a re-evaluation by EFSA. A second trend is the potential development of advanced Nicotinamide Mononucleotide NMN formulations. Future products may focus on improved bioavailability through advanced delivery systems or in combination with other synergistic molecules, such as Resveratrol or TMG (Trimethylglycine).
In conclusion, Nicotinamide Mononucleotide represents the cutting edge of nutritional science aimed at healthy aging. Its use in Europe is currently constrained by a cautious regulatory environment that prioritizes proven safety. While its proposed functions in boosting NAD+ are scientifically plausible, leading to its popularity in global markets, its legal pathway as a consumer health food in the EU is currently blocked. The future of Nicotinamide Mononucleotide NMN in Europe, therefore, hinges unequivocally on the scientific community’s ability to provide the high-quality clinical evidence required to satisfy regulatory standards, paving the way for its potential safe and legal integration into the European wellness landscape.
RDHealthIngredients offers super quality Nicotinamide Mononucleotide NMN with stable supply, compattive price. Are you looking for certified origin, traceability or safety? We propose a full range of titrated extract in accordance with GB, USP, EP, Pls find the list here. If not listed, please get contact with us at sales06@health-ingredients.com.
Or you can search them by A-Z Product List.


发表回复